What’s Involved
As part of RECOVER, patients with CF will undergo several tests at baseline and then at regular intervals. These tests include:
Lung Clearance Index
LCI is a simple breathing test involving normal breathing through a mouthpiece. It involves breathing in 100% oxygen for a few breaths and then measuring the gases that are breathed out to determine if there is any slowing down of airflow in the lungs. The test takes about 20-30 minutes overall as the procedure is repeated three times for accuracy. LCI will be performed at baseline and then every 6 months for 2 years.

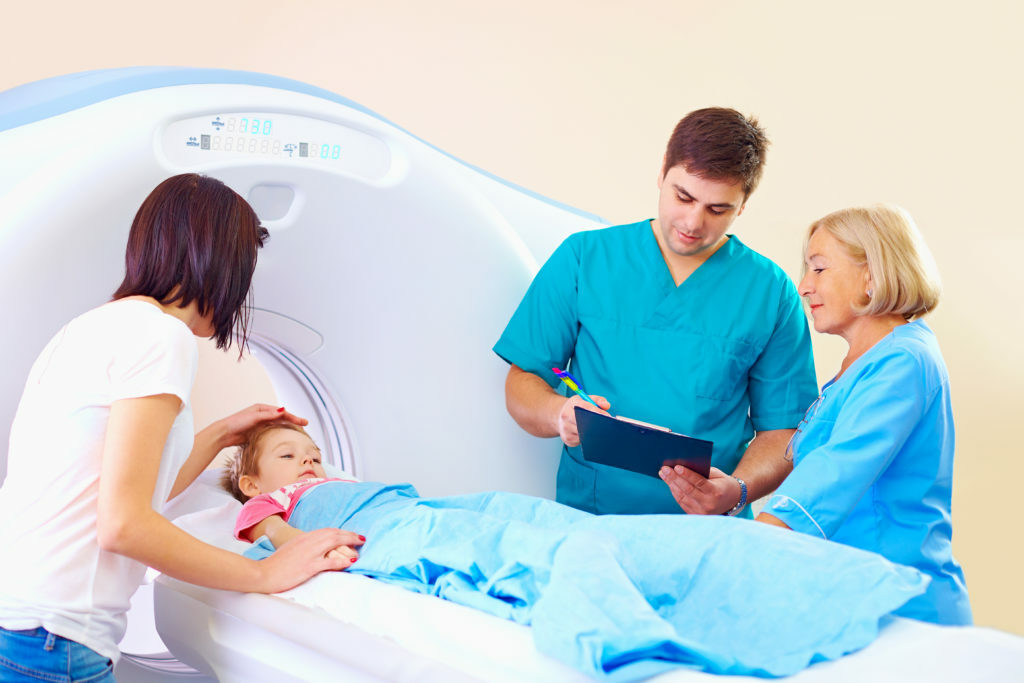
Ultra-low dose, spirometry-controlled CT scanning
A CT scan is a detailed three-dimensional image of the inside of the lungs and airways. It allows us to collect detailed information in relation to CF lung disease. CT scans are extremely helpful in CF but are associated with radiation, so they must be used carefully over a person’s lifetime. The type of CT needed in CF can be performed with substantially less radiation than a standard CT scan. Many centres around the world perform low dose CT scans every two years in people with CF as part of annual assessment. Our partners in the LungAnalysis Core in Erasmus, Rotterdam have been working with hospitals around the world to standardise CT scanners, advise on ultra-low dose protocols and analyse the results. LungAnalysis has worked with each centre in RECOVER to standardise scanners and help keep radiation doses down. Scans will be needed for RECOVER at baseline, year one and year two. Scans that are used as part of standard care can be used for RECOVER.
Sweat Chloride
Sweat chloride is a painless procedure and will be performed on both arms. Two small electrodes are placed on the arm with a gel disc for 5 minutes to stimulate sweating. Then a small sweat collecting “watch” is placed on the arm to collect the sweat. This takes roughly around 45 minutes in total to complete (30-minute sweat collection time per arm). This will be performed at baseline, at 6 months, 1 year and 2 years. Sweat will be analysed on samples using a specialised machine at each centre. This information will then be input into the database by the CF researcher.
Nasal Lavage
Nasal lavages will be performed on participants 12 years and at baseline, 6 months and 1 year. Participants aged 6 to 11 years will be asked to perform a nasal lavage if they receive this as part of their normal care, are willing to be recruited into the Sinus Disease cohort, and normally attend either CHI at Crumlin, CHI at Temple Street or CHI at Tallaght. These will be performed at Kaftrio® initiation, 6 months and 1 year. A nasal lavage is a technique in which the nasal cavity is washed to flush out mucus from the nose and sinuses. 10mls of sterile saline will be slowly washed into each nostril with the participants head reclined. This stays in the airways for 10 seconds before the participant leans forward and allows the saline to drain into a collection container. This is a painless assessment that takes about 5 minutes to perform. The participant will be coached on the technique prior to sample collection by the CF research coordinator/nurse. This will allow us to track nasal inflammation through RECOVER by measuring inflammatory mediators and any bacterial that may be present within the lavage.
Exhaled Nitric Oxide (FeNO)
Nitric oxide (NO) is an important molecule produced by airway cells that is related to inflammation and defense against bacteria. FeNO has been shown to be abnormally low in people with cystic fibrosis. The CFTR modulator Ivacaftor has been shown to lead to a significant increase in levels of FeNO in people with CF. We are therefore interested to see the impact Kaftrio® may have on FeNO measurement. FENO will be measure with the Niox VERO device via a mouthpiece during normal relaxed breathing. The study coordinator will train subjects on how to perform this. This assessment will take less than 5 minutes.
Sinus Disease (Sub-Study)
Participants aged 6-11 years old attending CHI at Crumlin, CHI at Temple Street or CHI at Tallaght will be invited to take part in the sinus disease sub-study. This will evaluate the effects of Kaftrio® on sinonasal symptoms, imaging and infection. Nasal swabs and questionnaires will be obtained from all participants in this sub-study and MRI scans will be required from up to 20 people. The Sinonasal-5 is a quality-of-life questionnaire that takes less than 5 minutes to complete and assesses parental report of sinus symptoms. Questionnaires will be delivered at baseline, 6 months and one-year post commencement of Kaftrio®. One swab from each side of the nose will be taken at baseline, 6 months and 1-year post Kaftrio® initiation.
MRI scanning (Sub-Study)
An MRI provides a detailed three-dimensional image which we can use to look at the sinuses that connect with the nasal space. An MRI Scan uses a magnet and radio waves instead of x-rays, thereby avoiding harmful radiation. The scan will take between 10 and 25 minutes. It is important that your child does not move during the scan because this can affect the quality of the pictures. Up to 20 participants aged 6-11 years old participating in the Sinus Disease sub-study will be invited to have MRI scans at baseline and 1-year after starting Kaftrio®. These scans will be performed in the Centre for Advanced Medical Imaging (CAMI) in St. James’s Hospital. CAMI have been established since 2008 and have expertise with performing scans in paediatric populations. A RECOVER researcher will accompany you on your visit to CAMI. Appointments for MRI scans will be organised around clinic visits where possible, or at a time that suits you and your child. Transportation to and from clinic visits will be organised free of charge. The Sinus Disease sub-study lead, Dr Gavin Stone, will be available to answer any questions you may have about this assessment. You can contact Dr Stone at gavinstone@rcsi.ie, or alternatively at CHI at Crumlin through the hospital switchboard.
Breath Sample Collection (Sub-Study)
A breath sample will be collected using a method designed by our collaborators in Amsterdam University Medical Centre and will be used to provide an insight into the bacteria growing in the lungs. It is largely unknown how the respiratory bacterial environment will respond to Kaftrio®. We would like to collect this breath sample on a small group of children aged 6-11 years old in this study (50 subjects across 4 clinical sites) to track changes in the lung bacteria before and after Kaftrio® start. Prior to breath collection, the participant will breathe normally through a mask (similar to a nebuliser mask) for 10 breaths. At the end of the 10th breath, your child will take a long slow breath in, and a long slow breath out. On the exhale, we will connect a bag to the other end of the mask to collect the sample. This takes 5 minutes to perform and will be collected at baseline, 3 months, 6 months, 9 months, 12 months and 24 months. This collection will only take place in Guy’s and St. Thomas NHS Foundation Trust and Children’s Health Ireland sites.
Liver Ultrasound
As per routine clinical practice, most people with cystic fibrosis from 6 years of age onwards have annual liver ultrasound scans as part of annual assessment. An ultrasound is a painless procedure that takes less than 10 minutes to perform. The ultrasound scan will be performed at baseline and at one and two years after commencement on Kaftrio®. We will link in with the radiology department in each hospital to collect the relevant information when routine scans are being performed, so repeat tests for RECOVER are not necessary. For centres where annual ultrasound is not performed every year, we will ask that a scan is performed each year for RECOVER.
Sputum Collection
As part of standard clinical care, sputum or throat swabs will be collected and processed in clinical laboratories. We will collect the information on the results and upload it onto our database. Separate to this, sputum samples for research purposes will be collected either spontaneously or after hypertonic saline induction (where possible) at study visits for measurement of markers of inflammation. Sputum for inflammation will be required at baseline, 6 months, one year and two years.
Stool sample collection
Inflammation in the gut can easily be measured in stool samples. A stool sample will be collected from study participants at baseline, 1 month after, 6 months after and 2 years after commencement on Kaftrio®. Sample collection equipment will be provided in advance to subjects and parents, so that samples can be collected on the morning of a study visit.
Abdominal Symptom Score
Our collaborators in Brandenburg, Germany have developed a specific abdominal symptom questionnaire for people with CF. The CFAbd score is validated in children and adults with CF and scores correlate well with ultrasound findings and gut inflammation. The CFAbd score has been used in studies in leading international centres. This questionnaire will take 5-8 minutes for the first time and 3-5 minutes on subsequent occasions. Questionnaires will be delivered at centres by the local research coordinator. The questionnaire will be collected at baseline, 1 month, 2 months, 6 months, 1 year and 2 years for all subjects.

Cystic Fibrosis Questionnaire – Revised (CFQ-R)
Over the last few decades a growing recognition of the importance of patient reported health related quality of life indices has developed. These indices are now recognized by patient organisations, clinicians and regulators as a vital tool in the development and testing of new treatments in people with cystic fibrosis. The Cystic Fibrosis Questionnaire-Revised (CFQR) is a specifically designed quality of life questionnaire for people with CF. We will utilise CFQ-R and the CFQ-R treatment burden questionnaire in this study. The CFQ-R treatment burden questionnaire is used to assess treatment burden in people with CF and assess to what extent do treatments make daily life difficult; how much time is spent on treatments and how difficult it is to do treatments every day? CFQ-R, including the treatment burden subscale, will be delivered at baseline, 6 months, one year and two years.
Pharmacy and MEMs Caps
As part of assessing adherence to treatments, prescribed medications at an individual level will be tracked throughout the study. A number of methods will be used to monitor and understand treatment adherence including electronic medication event tracking systems (MEMs), self-reported adherence and medication possession ratio (MPR) based on pharmacy pick up rates. MEMs is the gold standard for electronic monitoring. It mimics a traditional pill bottle with the enhanced ability to track the date and time of each bottle opening. Adherence to Kaftrio® will be measured with MEMs in a subset of subjects in Dublin centres. A feedback questionnaire will be provided if you or your child choose to discontinue using the MEMs. Self-reporting adherence will be collected using an up to date version of a specifically designed adherence scale for children and adults with CF. Subjects will complete a treatment adherence questionnaire, an adherence barriers questionnaire, while the study team will complete a prescribed treatment plan. The study also includes collection of pharmacy records from each subject’s community pharmacy. We will ask that you help us by asking your pharmacist to fax in your child’s pharmacy records to your local hospital. The researcher will remove all personal details from the record (name, address, DOB etc.), replace it with a code, and save it to the database so that our central pharmacy researcher in Dublin can work out what proportion of medicines that were taken prior to Kaftrio® starting and on treatment. This is the only purpose of reviewing pharmacy records. They will not be shared with the local CF Team (just the RECOVER researcher).
Interview about your child’s experiences of commencing Kaftrio® (Sub-Study)
A small cohort of 20 participants aged 12-17 years old will be invited to take part in an interview to tell us more about their experiences since starting this new treatment in Children’s Health Ireland sites. We will be asking how people with CF feel about Kaftrio®, how it affects people’s quality of life and what they expected from this treatment compared to how it really is. Interviews help us to understand more fully what it is really like for the person and allows their voice to be included in the research findings. The interview will take place at the 12-month visit and should take about 45 minutes to 1 hour to complete. This will be scheduled to suit your child, either in the clinic or else on a video call, using Microsoft Teams. Some of the questions will be about quality of life and how they manage their CF treatment. They will also be given some short questionnaires (Patient Health Questionnaire-9 and Generalised Anxiety Disorder-7) that ask questions similar to what is outlined above. The interview will happen approximately at the 1-year point.
Interview about your child’s health beliefs about CF and its related treatments
This part of the study will provide insight into what your child understands about their condition and the treatments they receive. A small group of participants aged 6-11 years old will be invited to take part in a brief interview which will include open ended questions and drawings. We will ask your child questions about their knowledge of CF, what helps, what gets in the way, and how CF makes them feel. Your child’s answers will not be corrected by the researcher. Your child will not be given any new information about CF. Interviews will take about 30-45 minutes and can be facilitated in clinic or on a video call, using Microsoft Teams. If your child mentions anything about their experience of CF that indicates that they are struggling to cope emotionally or practically, or to understand their condition, we will flag this to you as their caregiver and the treating team. We will ask that you will also answer some short questionnaires to explore your own beliefs about CF and how they relate to your child’s health outcomes. The questionnaires include: Patient Health Questionnaire-9, Generalised AnxietyDisorder-7, the Illness Perceptions Questionnaire-Revised, the Beliefs about Medication Questionnaire. The interview and questionnaire administration will take place at the 6-month point of the study.
Other clinical parameters in medical notes
Other routine health measurements will be collected from subject’s medical charts with consent. Data collected will include the following:
⦿ Lung function measurements
⦿ Liver blood tests
⦿ Nutritional measures such as height, weight and BMI
⦿ Airway sample microbiology results
Not all assessments will be performed by all subjects. There is a standard and an advanced treatment arm for the study, as well as several sub-studies being run in parallel with RECOVER. Your local clinical research coordinator or study team member will discuss these with you at recruitment and will determine if you are eligible to participate.
For more information about RECOVER and the assessments involved please contact us at info@RECOVERCF.ie.
